RESEARCH

Introduction
First of all, let me introduce you about the “antibodies” that we are focussing on.
Antibodies are proteins that exist in the body fluids of animals (mainly in blood) and specifically bind to foreign substances (antigens) that have entered the body from outside.
Currently, antibodies are utilized as medicine, diagnostic agents, and affinity ligand of chromatographic resins because of their exceptional ability to distinguish antigens and the others from complicated biomoleculmixture and their extremely high binding affinity (binding strength) to antigens.
The binding of antibodies to antigens is significantly specific that it is often well-known as a relationship between key and keyhole. It is said that tens to hundreds of millions, or even more, of different types of antibodies potentially exist in the bodies of animals, and their superior function and diversity keep our bodies healthy.
In other words, our bodies are monitored by a large number of antibodies against unknown foreign enemies that may invade at any time from the outside. The leukocytes recognize the antibodies that are binding to the antigen. The leukocytes then gather around the antibodies and rapidly remove the foreign antigens from the body.

Therefore, it would be thanks to antibodies that we are able to live healthy on this planet.
These exceptional and adorable antibodies are not only useful in our bodies. Antibodies are used as diagnostic agents for a variety of infections diseases such as influenza and new corona viruses, as medical drugs for serious diseases such as cancer, rheumatism, and Alzheimer’s disease, and as affinity ligands in the industrial production of various biopharmaceuticals processes, and researchers around the world are continuing various challenges with the expectation of further development. In addition, researchers around the world are continuing various researches in anticipation of further development of antibody engineering.
Our group is actively engaged in research to extract, nurture, and maximize the antigen recognition ability antibodies, which are such a behind-the-scenes force, and return it to society.

The Impact of Research on Society
The benefits of our research to society include the ability to detect diseases a little earlier, shorter waiting times at the hospital, and slightly lower hospital bills.
Many other researchers besides us are doing it all over the world. If you think globally, you can imagine that society will be dramatically improved by these daily efforts.
And on a global scale, tens of thousands of lives are being saved by “small changes”. We are happy and proud to accumulate such small results, and enjoy our research activities every day.

One of our researches
Now, we will briefly explain one of our researches, diagnostic agent development. This may be a bit difficult for non-specialists as it contains some technical expressions.
Anyone who would like to ask about something they don’t understand or would like to know more about it, please feel free to contact us through the “Contact Form“. We also welcome those who would like to know what kind of efforts we are making on research themes other than reagents.
We also recommend you to check our research database, Lablogs, for the latest research results and initiatives. (You must be a registered user to access Lablogs 1-3. Unregistered users can only view Lablog4 and Lablog5).
Antibody Functionalization in Clinical Diagnostics
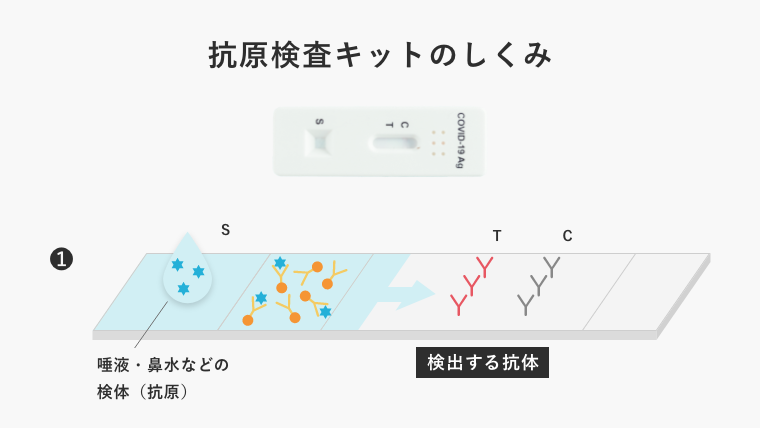
A example of how antibodies work is the antigen test kit for new coronavirus infection.
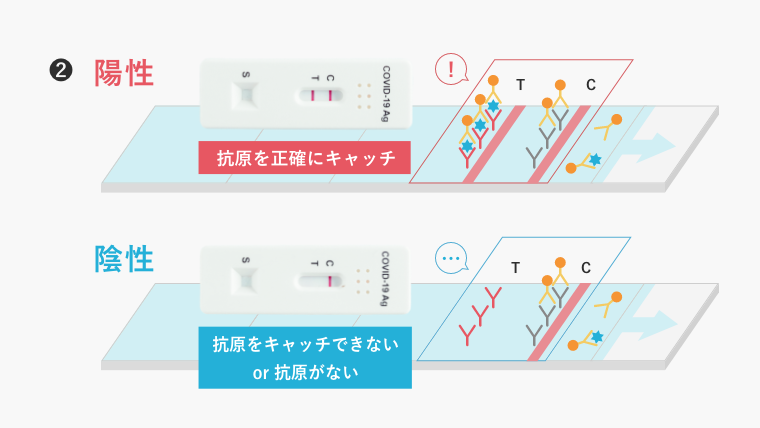
The material substrate of the test kit is lined with antibodies that catch the antigen of the virus (generally Y-shaped). When saliva or other substances are dropped onto this substrate, if the antibodies catch the antigen, the test is considered positive; if not, the test is considered negative.
If the antibodies themselves are of low activity, they fail to catch the antigen, resulting in a low test accuracy.
Innovative technology that will change the future of clinical diagnosis
The antibodies used in current test drugs are mainly manufactured from animals, making it difficult to modify their molecular structure and physical properties, resulting in “high development costs and low test accuracy. (There is also the ethical problem of using animals.)
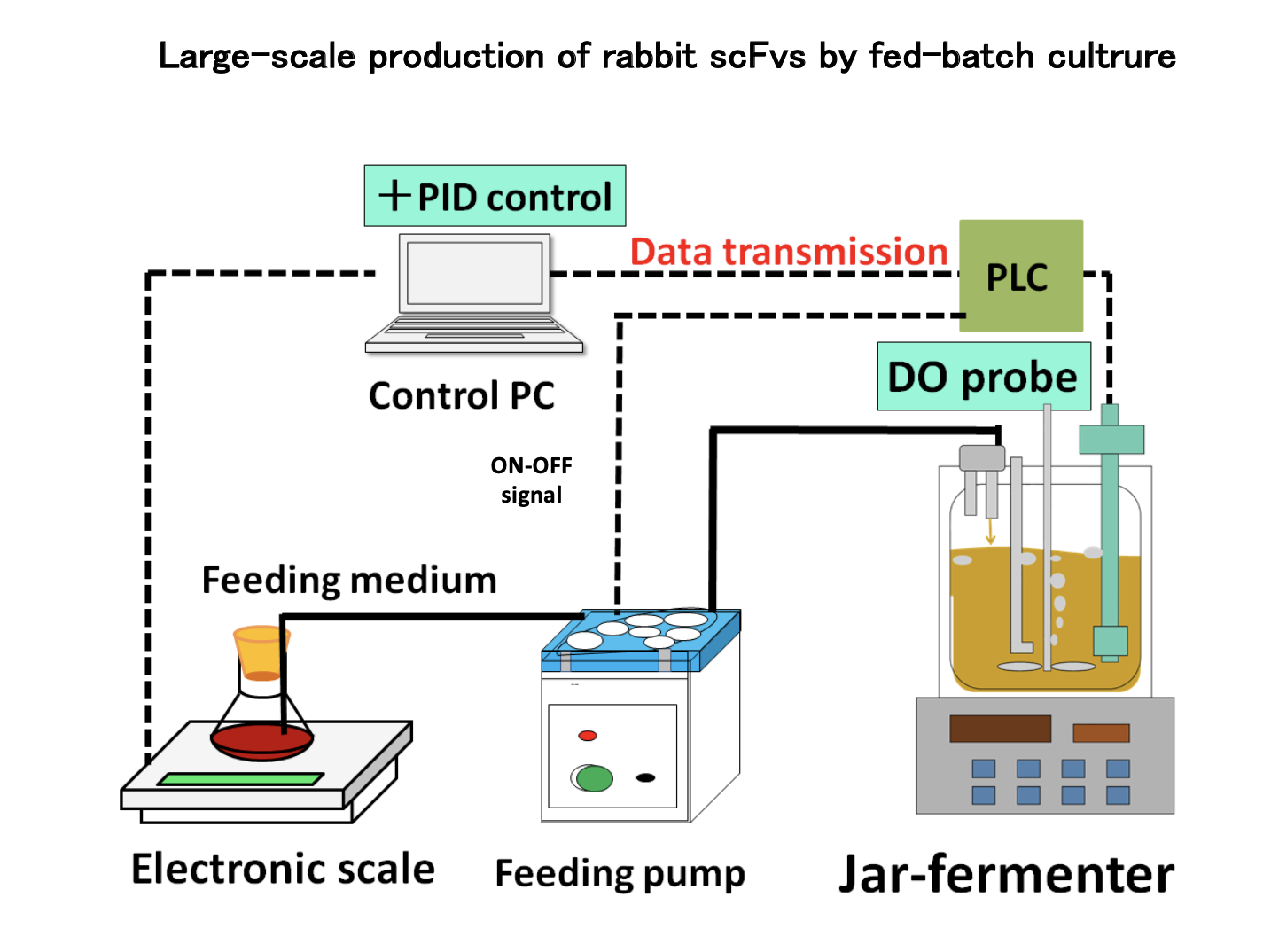
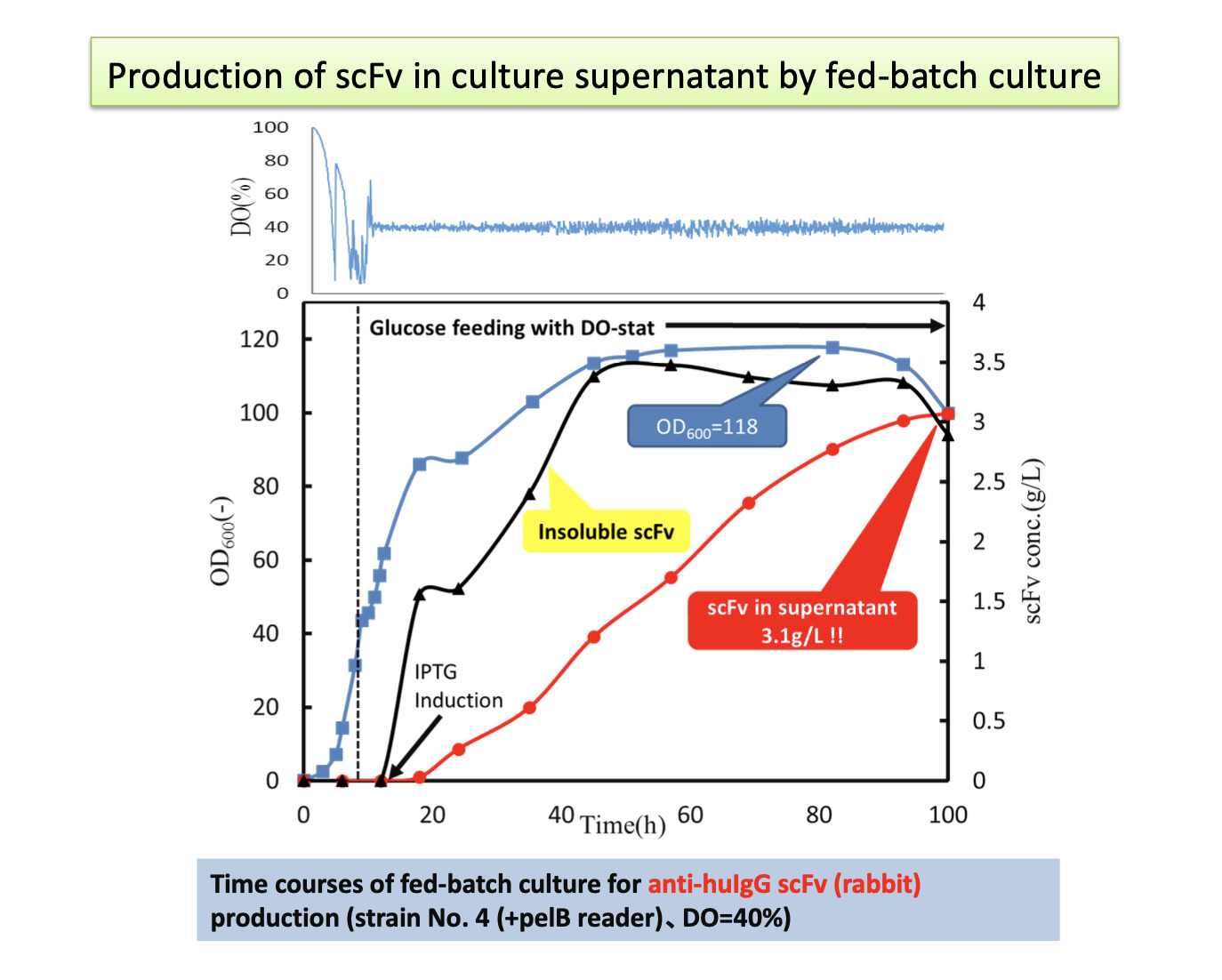
Therefore, we are developing completely new antibodies that can significantly improve testing accuracy and enable low-cost, mass-production without the use of laboratory animals.
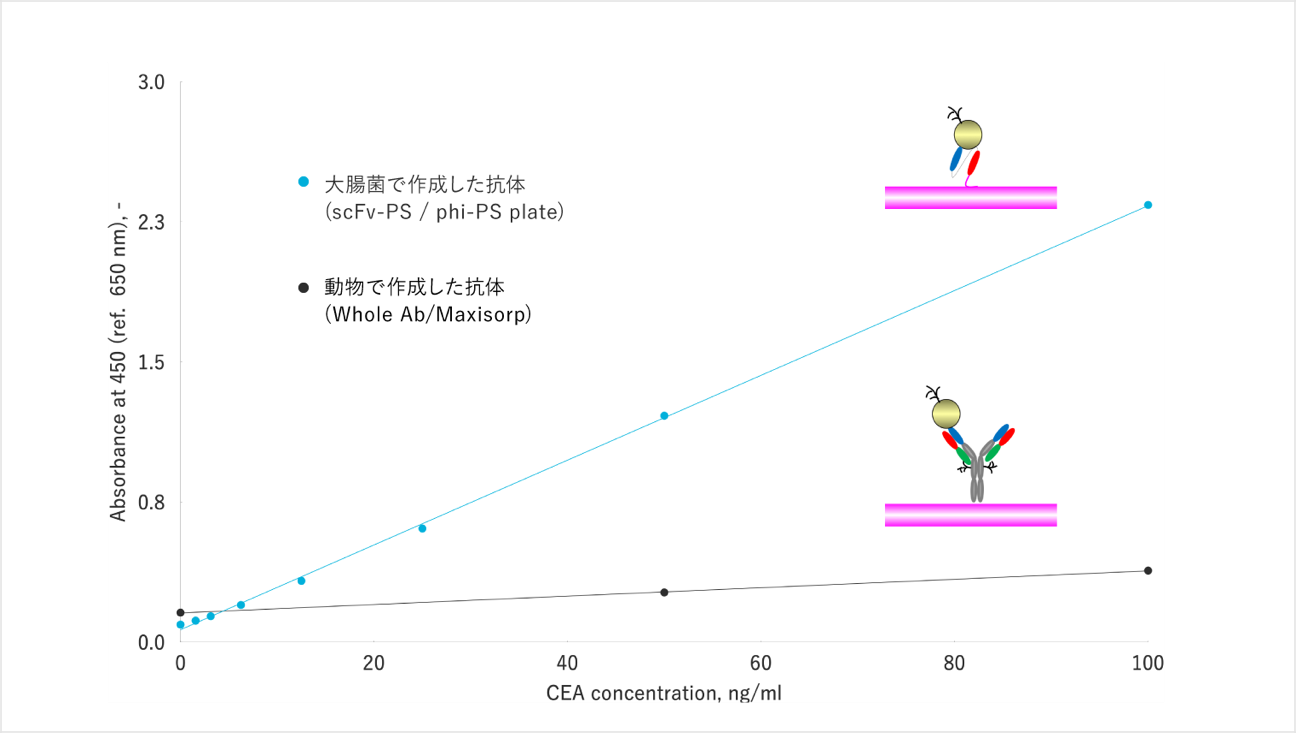
Please see the figure to see how much difference in performance between the conventional and our new antibodies. The black line shows the results using conventional antibodies made from laboratory animals (whole antibodies), and the blue line shows the results using the antibodies we have developed. (single-chain antibodies).
The vertical axis is the inspection accuracy, and you can see that the performance is more than 10 times higher than that of conventional antibodies. This is because we have intentionally modified the antibody molecule to suit the application so as to increase the inspection accuracy.
Development of Next-generation of Diagnostic Agents
We would like to introduce the processes that we have developed our new diagnostic agents.
The first step is to select and prepare a good antibody that binds to the target antigen.
1. Extraction of 100 antibody candidates from more than 100 million antibody repertories.
2. Those 100 different antibodies are analyzed from all perspectives and narrowed down to one.
This step is like a treasure hunt. In this process, you will encounter unexpected discoveries and possibilities.
The antibodies selected in step 1 are outstanding in their ability to bind to antigens, but there may be also many downsides. Therefore, they can rarely be used as diagnostic agents. (Difficulty in mass production, difficulty in preservation, etc.)
Molecular modifications are investigated to overcome the above disadvantages while maintaining the “antigen-binding ability” of the antibody.
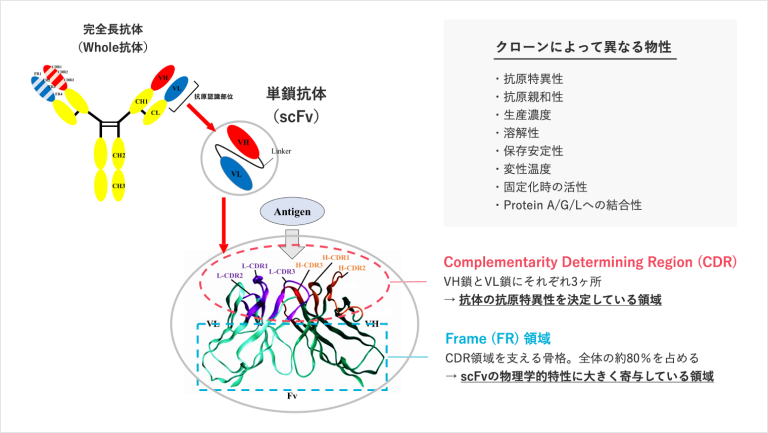
【Technology 1: Frame switching technology to create new functional antibodies】
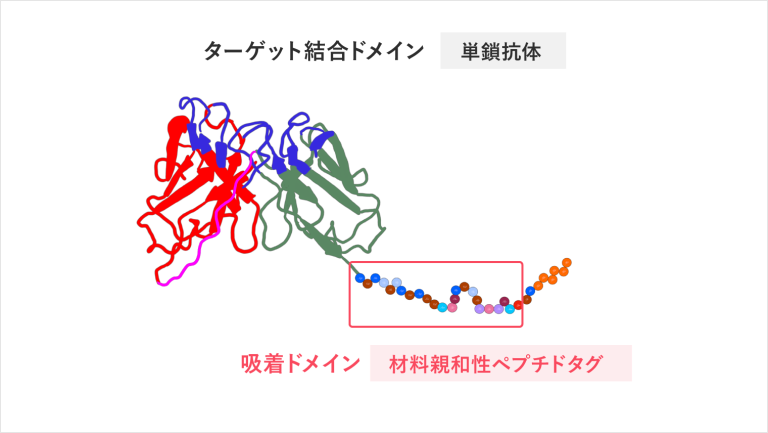
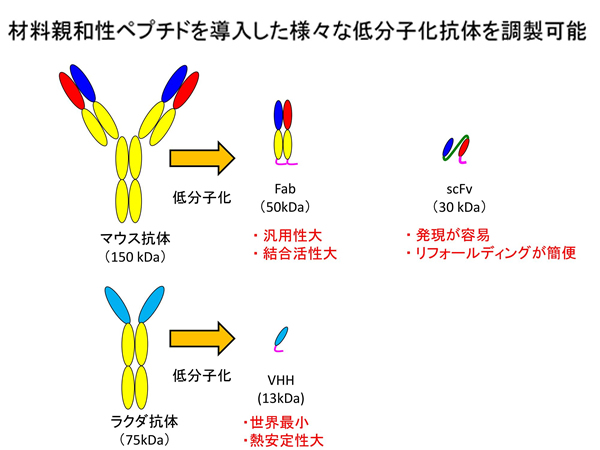
We divide an antibody into two parts: CDR, which binds to the antigen, and FR, which is the backbone of the antibody. We create new functional antibodies by molecularly modifying the binding part and the frame.
For example, if the single-chain antibody discovered in step 1 has binding strongly to the antigen but cannot be produced at higher level, it is possible to modify the frame (FR) of the backbone and change its characteristics to enable mass production while maintaining its binding strength.
Conversely, by replacing the binding part, the antigen to which it binds can be changed, and it is even possible to change it into a different diagnostic agent.
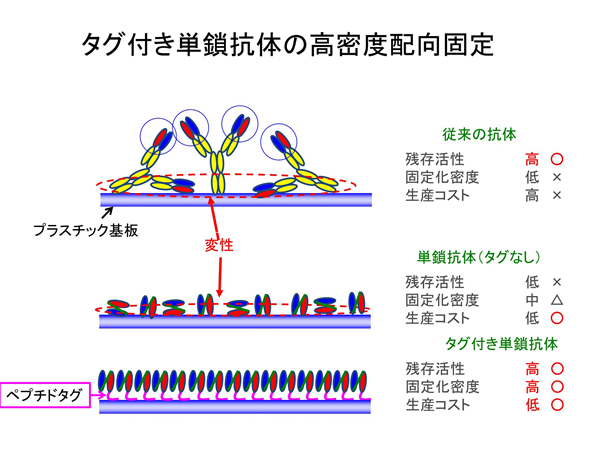
As shown in the picture, all antibodies of the test reagent are immobilized on a material substrate for use. Therefore, the point for sensitive diagnostics is “how densely and actively the antibody can be immobilized on the material substrate.
The conventional antibodies, which are the current mainstream of testing reagents, are Y-shaped, and the area circled in red is the part where the antibody binds to the antigen. The two binding sites are supported by one leg (Fc region).
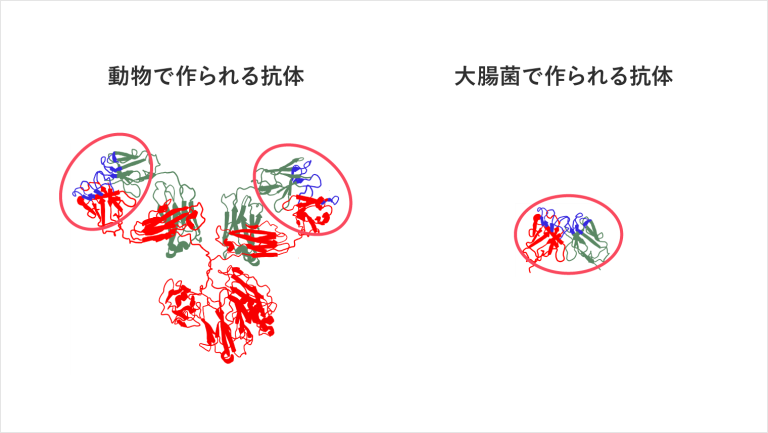
This conventional Y-shaped antibody is very unstable to keep upright on the substrate and is rather prone to collapse sideways, rarely lining up in an ideal molecular orientation. In addition, there are many areas other than those necessary for binding to the antigen, making it impossible to lay down the antibodies in a dense layer. These are the reasons for the low accuracy of the test.
However, it is difficult to further improve the molecular orientation of the antibody, and thus there is nothing more that can be done.
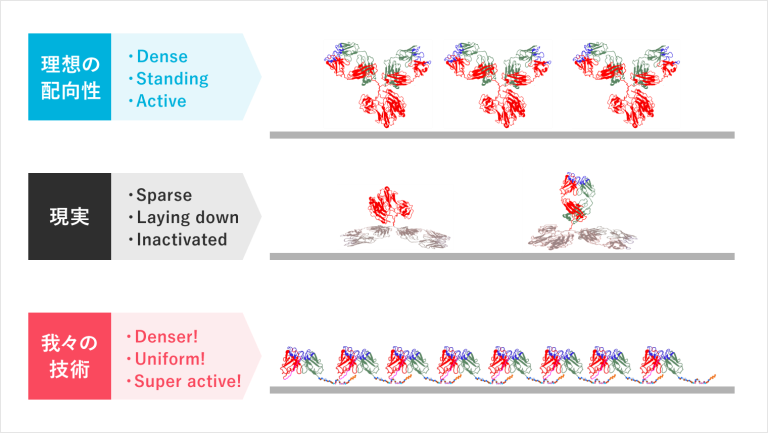
【Technology 2: Using materials-binding peptide tags to connect between substrate and antibody】
The single-chain antibodies we are developing are much smaller in molecular size than the conventional antibodies and can be densely immobilized on the surface of materials. Furthermore, an adhesion region called a materials-binding peptide tag can physically link the antibody itself to the surface of the substrate material.
This technology is very unique and has many possibilities.
Rather than “improvement of antibodies,” it is more like “research to create new proteins that do not exist in the world,” and this research goes beyond the development of antibodies and test drugs. Both scFus and materials-binding peptide tags are sequences that exist in nature, but a peptide tag-fused scFv, which is a combination of a scFv and a peptide tag, is a new protein that does not exist in nature. Also, depending on the type and shape of the material substrate, we can develop the best materials-binding peptide tag suitable for it.
In order to perfect the immobilization of antibodies, it is also necessary to take on the great challenge of protein engineering to design and create new proteins.
Document
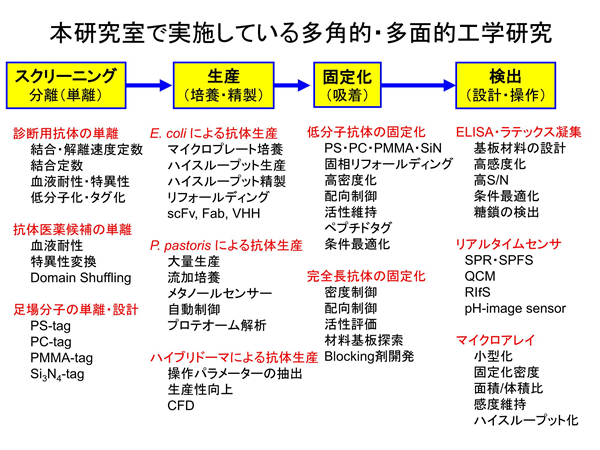
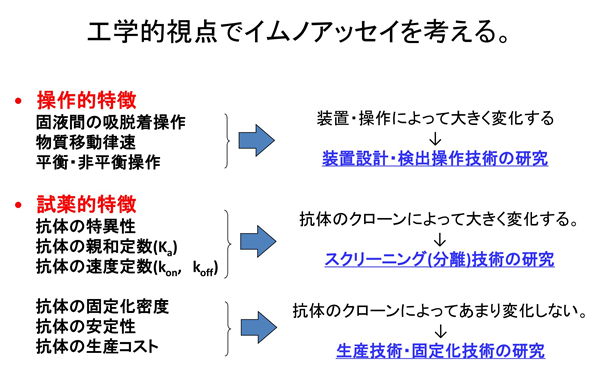
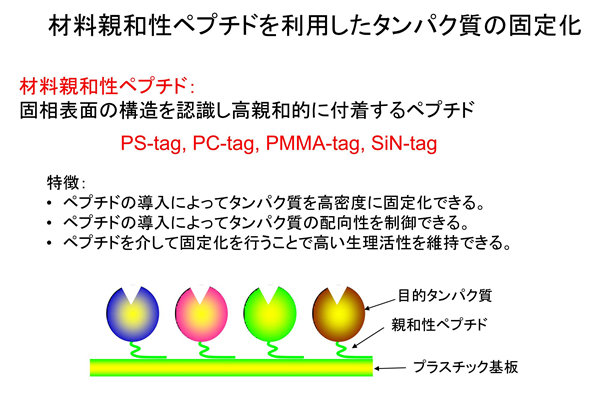
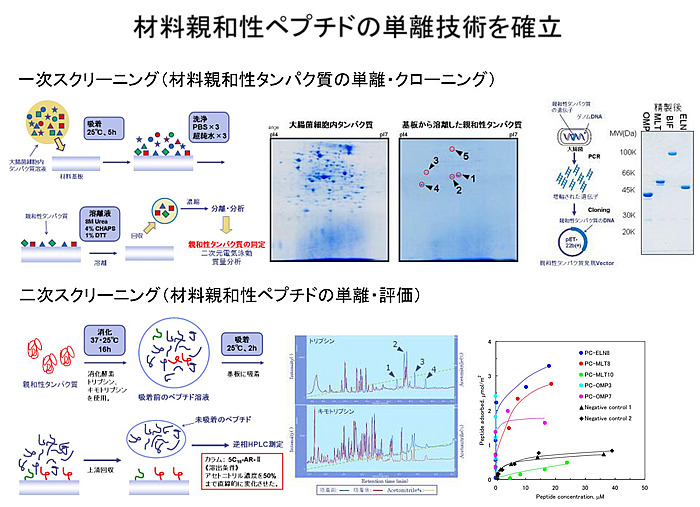
Industry-University Cooperation
Whether you are interested in revolutionizing the bioaffinity field, using our original technologies, or learning more about the technology, we open a wide range of collaborations including joint research, academic lecture, and advisory consulting. Please feel free to contact us.
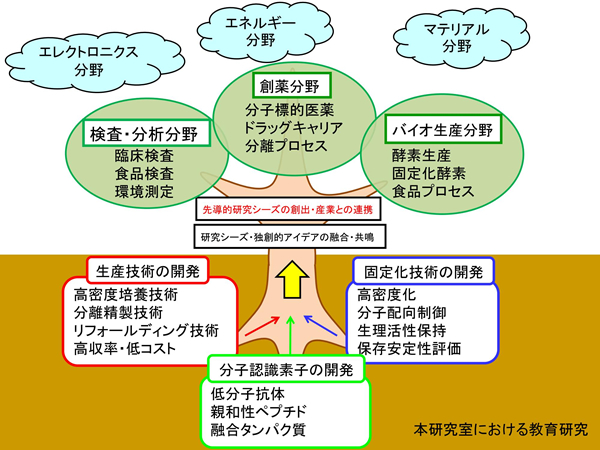
Our vision beyond research is to “create a platform for antibody technology that anyone can utilize. Our goal is that anyone with access to this platform technology will be able to design, improve, and manufacture antibodies suitable for test drugs and affinity ligands, and anyone will be able to create highly functional affinity molecules and affinity carriers. This may be likened to a platform such as a book or website that contains a comprehensive collection of cooking recipes.
We also hope that a variety of technologies will come together on this platform, transcending the boundaries of the fields of biochemical engineering and antibody engineering. This is because the final goal of my research is not to “create perfect antibodies,” but to “create perfect affinity carriers” beyond that point. Then, there is more than just antibodies to tackle. It is deeply related to all fields of research, including materials science, nanotechnology, and data science.
We hope that this platform will remain in the next era when we are gone, and will be further developed as other researchers add new methods. This may sound a bit, well, big, but I am convinced that the reason I am working on antibodies now is to make this platform a reality.

CONTACT US!
Please feel free to contact us.
